|
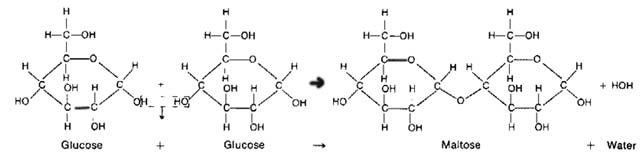
Dehydration synthesis and hydrolysis concepts are easy to understand, mainly due to them being easy reactions and the names are easy to understand. Dehydration means to take water out. Theus when you use dehydration synthesis, you are building something up while taking water out. In carbohydrates, an H from one carbohydrate and an OH from another are taken out. They form water. The two carbohydrates are then joine together by a bond called a glycosidic linkage. Hydrolysis is simple the reverse of dehydration synthesis. You add water to a molecule to break it down. If dehydration synthesis continues for a long time, a long and complex carbohydrate chain called a polysaccharide is formed.
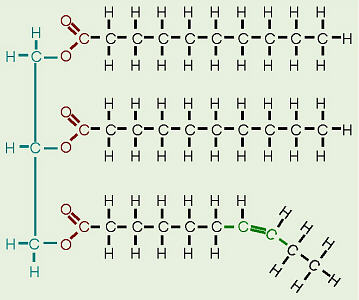
The molecule on the top is a lipid. It has been color coded to show the three main features. The green part shows what was the glycerol. It reacts with the purple parts (the 3 fatty acids) to form the lipid. The red part is where the dehydration synthesis occurs and a water molecule comes out. The red part bonds the fatty acids to the glycerol and this bond is called an ester linkage. Once again, hydrolysis is just the reverse of dehydration synthesis.
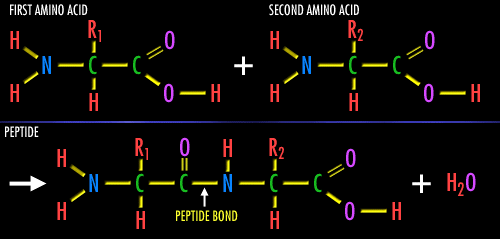
Dehydration in proteins involves the combination of two amino acids as shown. The water molecule comes off like the carbohydrate reaction. The bond in between the two amino acid molecules that forms is called a dipeptide bond. Just like all other dehydration synthesis reactions, the hydrolysis is the reverse.
|