|
What's a proton...pro-teen...protin...or whatever it's called?
Many of us tend to dislike beans, especially after we learn what type of smell they give. However, beans are an important source of proteins and proteins are probably the second most important food group after vabohydrates. They are so important because proteins are virtually everywhere in our bodies. They control and take part in almost every reavtion and metabolic process. For example, enzymes are a type of protein that are present in virtually all reactions of our body. Enzymes are catalysts and speed up and mediate the reactions in our bodies. Without enzymes, the reactions would have no meaning or be too slow and we would cease to live if they didn't function properly. Other uses of proteins are that they strengthen cells, store nutrients, transport (hemoglobin is a protein that is used to transport oxygen to out cells), regulate gene expression (hormones like insulin are proteins), and protect the body (antibodies). As you can see, proteins are very important for us. Another important thing about proteins is that our genetic information (our DNA) actually codes for proteins. In order for the DNA to be read by our cells, the DNA must first be "translated" into a protein chain.
Amino Acids are Your Amigos
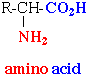
Proteins are made up of subunits called amino acids (see figure on left). The red portion of the amino acid is called the amino group and the blue is the acid or the carboxyl group. The R stands for a variable side chain. The R can be replaced by one of 20 different variables. Thus, there are a possible 20 amino acids. Our cells can make all but 8 of the 20 amino acids. Those 8 that must be taken in are called the essential amino acids.
Synthesizing a Bean
Amino acids join to each other by a process called dehydration synthesis. It is exactly the opposite of hydrolysis. What happens is that a hydroxide ion (OH-) group is removed from the carboxyl group of one amino acid and a hydrogen ion (H+) is removed from an amino group of another amino acid. Then, the two ions join to form water ("dehydration") and the two amino acids are joined together by a dipeptide bond ("synthesis"). A long chain of amino acids would be a polypeptide chain.
|
|
         |
 |
|  |  |  |
|