|
Lipids are hydrophobic organic compounds. Because they're hydrophobic, they tend to be soluble in nonpolar solvents. Fats, phospholipids, carotenoids, steroids, and waxes are some types of lipids. Lipids have varied uses. They're used for energy storage (when in need of energy, the body can resort to using body fat which has energy stored in it), make up the cell membrane, and are also used as hormones.
The most abundant types of lipids are triglycerides (known as fats in everyday life). They're used as energy reserves and yield twice as much energy as do carbohydrates. The name might suggest that a triglyceride is made up of three glycerols. However, that is not the case. It is actually made up of one glycerol unit with three fatty acids attcahed to it (see picture below, right.). THe link between the glycerol and the faty acid is called an ester linkage.
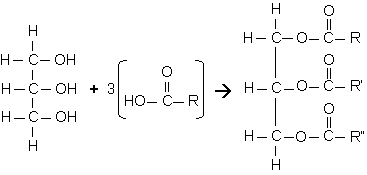
|
| Rxn. of glycerol (left) and 3 fatty acids (middle) to form a triglyceride (right). |
When we eat food and look at the nutrition facts (like we ever do), one can see that the fats are broken up into saturated and unsaturated. Saturated means that the fatty acid contains the maximum number of hydrogens that it can hold to all its carbons. Thus, the fatty acid is "saturated" with hydrogens. In other words, there are only single bonds between carbons of a saturated fatty acid. Saturated fats tend to be solids at room temperature because of the presence of intermolecular bonds called van der Waals forces.
Unsaturated fatty acids have double or triple bonds between the carbons. For this reason, they don't have the max number of hydrogens. The double and triple bonds make the shape of an unsaturated fatty acid twisted and bent. For this reason, the van der Waals forces are less effective and the unsaturatd fatty acid is a liquid at room temperature.
Another type of lipid is the phospholipid. It belongs to a group of lipids called amphiphatic lipids. These are lipids that hydrophilic (like water) at one end and hydrophobic (hate water) at the other. The structure of the phospholipid is the same as any lipid except that the phospholipid has a phosphate group replacing one of the fatty acids to the glycerol. The phosphate group is attcahed on the other side of the fatty acids and has another organic compound bound to it. The part with the phosphate is called the "head" and is hydrophilic. The fatty acid chain makes up the "tail" and is the hydrophobic part.
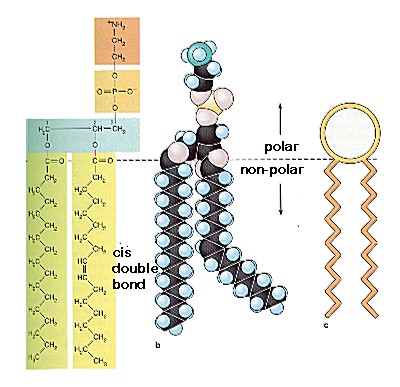
|
| The left shows structure of phospholipid. The middle, a 3D look. The right shows general picture. |
|