|
To understand organic compounds, it is essential to understand carbon because that's what organic molecules are mabe up of. Carbon, to most people, is just another element on the overcrowded periodic table. They look up and see number six as just another one of the 100+ numbers listed. Their eyes might as well just waver down to number 47 (gold). Although each element on the table is of importance and has a lot to reveal, carbon is one of the more important ones. It's true that we need water and oxygen to survive, but equally essential is carbon. We are referred to as "carbon-based life". In fact, the only type of life that we know of on Earth is carbon-based.
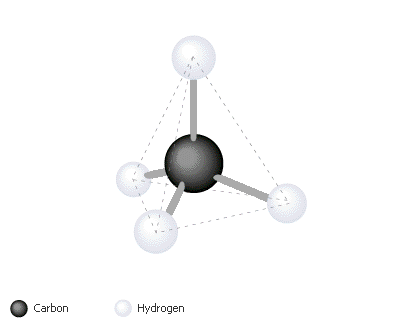
So, what makes carbon important for sustaining life? Well, many things actaually. One property of carbon is that it has four valence electrons. This means that, as the picture below shows, carbon can make four bonds with other atoms. If each carbon bonds with another carbon, strong carbon-carbon bonds are formed and a huge carbon chain is formed which can serve as a basis for formation of very large molecules. Carbon is not only confined to forming single bonds. It can form double or triple bonds too.
When carbon bonds to four atoms, the shape shown below (a tetrahedron) is formed. As more and more carbons join to each other and other atoms and as different types of bondings occur (single, double, and triple) the resulting molecules have diffrent shapes.Shapes are also important in determining how a biological molecule acts. Molecules of diffrent shapes have different properties, boilinf points, and so forth. Thus, molecules formed with carbon chains result in many diffrent varieties and functions.
 |
 |
|
|
Bonds away!
Carbon can make four strong bonds with many other elements. The four bonds attached to carbon make a tetrahedral shape.
|
|  |
 |
         |  |
 |
 |
|
Quick Carbon Facts
Atomic Number: 6
Average Atomic Mass: 12.011
Melting Point: 3823 K (3550C or 6422F)
Boiling Point: 4098 K (3825C or 6917F)
Density: 2.267g/cu.cm.
Stable Isomers (2)
Standard state: solid at 298 K
Color: graphite is black, diamond is colourless
Classification: Non-metallic
Availability: carbon is available in several forms including amorphous, powder, graphite rods, diamond, "bucky tubes", foil, sheet, and wire.
|
|
|
|